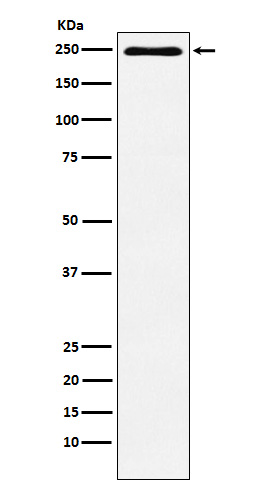
产品名称
Plexin A1 (3J3) Rabbit Monoclonal Antibody
别名
NOV; NOVP; Plexin-A1; Plexin1; PlexinA1; PLXN1; Plxna1;
纯度
Affinity-chromatography
存储缓冲液
Rabbit IgG in phosphate buffered saline , pH 7.4, 150mM NaCl, 0.02% New type preservative N and 50% glycerol. Store at +4°C short term. Store at -20°C long term. Avoid freeze / thaw cycle.
Human Swissprot No.
Q9UIW2
注意事项
Plexin A1 Antibody is for research use only and not for use in diagnostic or therapeutic procedures.
组织表达
Detected in fetal brain, lung, liver and kidney.
细胞定位
Cell membrane; Single-pass type I membrane protein
功能
Coreceptor for SEMA3A, SEMA3C, SEMA3F and SEMA6D. Necessary for signaling by class 3 semaphorins and subsequent remodeling of the cytoskeleton. Plays a role in axon guidance, invasive growth and cell migration. Class 3 semaphorins bind to a complex composed of a neuropilin and a plexin. The plexin modulates the affinity of the complex for specific semaphorins, and its cytoplasmic domain is required for the activation of down-stream signaling events in the cytoplasm (By similarity).