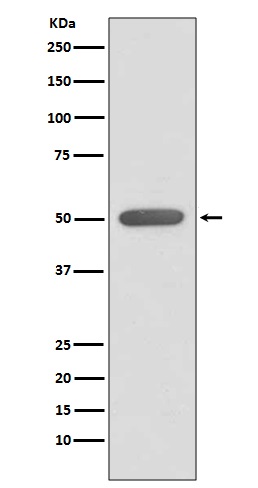
产品名称
ERR alpha (18W8) Rabbit Monoclonal Antibody
别名
ESRRA; ERRa; ERR1; HERR1; NR3B1; Steroid hormone receptor ERR1; ERR-alpha; ERRalpha; ESRL1; Estrogen receptor-like 1; Estrogen-related receptor alpha;
纯度
Affinity-chromatography
存储缓冲液
Rabbit IgG in phosphate buffered saline , pH 7.4, 150mM NaCl, 0.02% New type preservative N and 50% glycerol. Store at +4°C short term. Store at -20°C long term. Avoid freeze / thaw cycle.
Human Swissprot No.
P11474
注意事项
ERR alpha Antibody is for research use only and not for use in diagnostic or therapeutic procedures.
细胞定位
Nucleus {ECO:0000255|PROSITE-ProRule:PRU00407, ECO:0000269|PubMed:18063693, ECO:0000269|PubMed:21190936}. Cytoplasm. Note=Co-localizes to the cytoplasm only in presence of MAPK15.
功能
Binds to an ERR-alpha response element (ERRE) containing a single consensus half-site, 5'-TNAAGGTCA-3'. Can bind to the medium- chain acyl coenzyme A dehydrogenase (MCAD) response element NRRE-1 and may act as an important regulator of MCAD promoter. Binds to the C1 region of the lactoferrin gene promoter. Requires dimerization and the coactivator, PGC-1A, for full activity. The ERRalpha/PGC1alpha complex is a regulator of energy metabolism. Induces the expression of PERM1 in the skeletal muscle.